|
| |
Creative Logic Software, LLC
Would like to hear your thoughts…
Please contact us
or call?
Thanks!
Don Shave
|
|
| |
|

|
|
This page provides management methods that can consistently achieve regulatory compliance for medical imaging devices.
|
|
|
| Area |
Information |
| General |
Compliance is a critical and mandatory requirement for businesses that design and deploy certain kinds of medical imaging devices. These businesses are directed in the US by a formal legal entity, the
FDA, where product life-cycle management for such devices is controlled by federal laws; view an overview of the
21-CFR Part 820
law, as well as the FDA's details page.
Methods & processes may be directly derived from these laws; examples include such items as a new product introduction process (NPI), a disciplined phased product development process (PRD) and CAPA (see below) as well as others.
|
| Workflow |
Businesses such as these must use a
structured approach
for compliant product management:
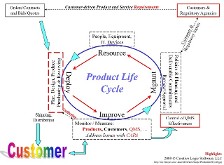
The red items (highlights) are listed below; click either the image or the link for a better view?
The list starts with the item that is at the top right-hand corner of the image…
|
|
Highlights
1.
|
The "Customers & Regulatory Agencies" highlighted box (top right) is the primary root (or the start point) of a product cycle, where the identified groups each bring data and information into the business, feeding the annual planning process (WWPP) and the new product introduction (NPI) cycles.
|
| 2. |
Requirements are the next phase of the cycle and form the "essential definition" of a product, covering all aspects of the system in a formal, approved document that is under revision control.
View expanded and more detailed definitions of this term here and here.
|
| 3. |
The "Plan, Design, Production, Purchasing and Receiving" box (PDPPR) that follows in the cycle is a conceptual encapsulation of the various areas of a business…
- An Engineering team (with the Marketing and Research teams) designs and deploys the product using a milestone-based process (such as the PRD), and
- Manufacturing then constructs the systems (with Purchasing & Receiving);
- Sales then move the systems on to the Customers where
- Service then manages the life cycle of the fielded systems.
|
| 4. |
Verification (definition) is a critical step in the sequence, and has bi-directional execution
When moving forwards (towards Monitoring & Measuring), V&V gates product release.
When moving back to PDPPR, V&V is part of the CAPA process.
|
| 5. |
Products
are essential–without a successful product, income ceases, the cycle dies & the business suffers from the "inconvenience" of huge profit gaps!
|
| 6. |
CAPA is the acronym for Corrective and Preventive Actions, the core of compliance issue resolution.
As an example of the significance of a miss here, GE's OEC business was shut down (news article; scroll down to the "GE Radiology Units Shuttered by FDA" section) for a very, very long time in 2007 from a repeated failure to comply with previous CAPAs…not a great idea.
View the legal definition of CAPA here and a summary here; the term "QSIT" is the FDA auditors guide.
|
| 7. |
A Quality Management System (QMS) is the backbone to allow a business to achieve compliance; view a sample QMS to see how a QMS might be constructed upon the 21CFR clauses.
The opening statement also establishes the overall intent:
<company>, part of <corporation>, strives to observe key attributes of the relevant medical world more clearly, thereby helping its customers to better understand many attributes of disease, and how such diseases are diagnosed & treated to help allow individuals to live life to the fullest.
|
| 8. |
Control of QMS Effectiveness is the gate that permits or stops the cycle from moving forwards.
|
|
Further refinements to come… |
|
|
|
| |
|
|
|
| |
|
|
| |
|
|
|

